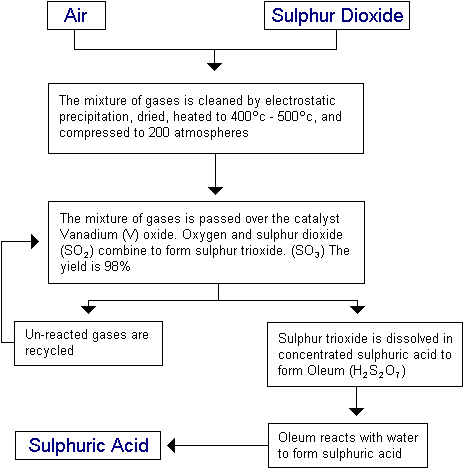
The contact process is the name given to the process by which sulphuric acid is produced. Sulphuric acid has many uses such as the manufacturing of:
- Paints / Pigments
- Soaps / Detergents
- Fibres
- Plastics
- Fertilisers
The following reactions are involved:
- S (s) + O2 (g) —-> SO2 (g) (sulphur dioxide)
- 2SO2 (g) + O2 (g) —-> 2SO3 (g) (sulphur trioxide)